The 3beLiEVe project goal was to develop next-generation automotive battery cells free of cobalt and natural graphite. As part of the assessment for compatibility with a circular economy, it is important to consider the cells’ ultimate end of life, following their use in automotive and, potentially, second life applications. Depending on their compositions, novel cell chemistries (e.g., those not containing cobalt) pose different demands on the recycling treatment. Therefore, in 3beLiEVe, an assessment of the cells’ recyclability was performed.
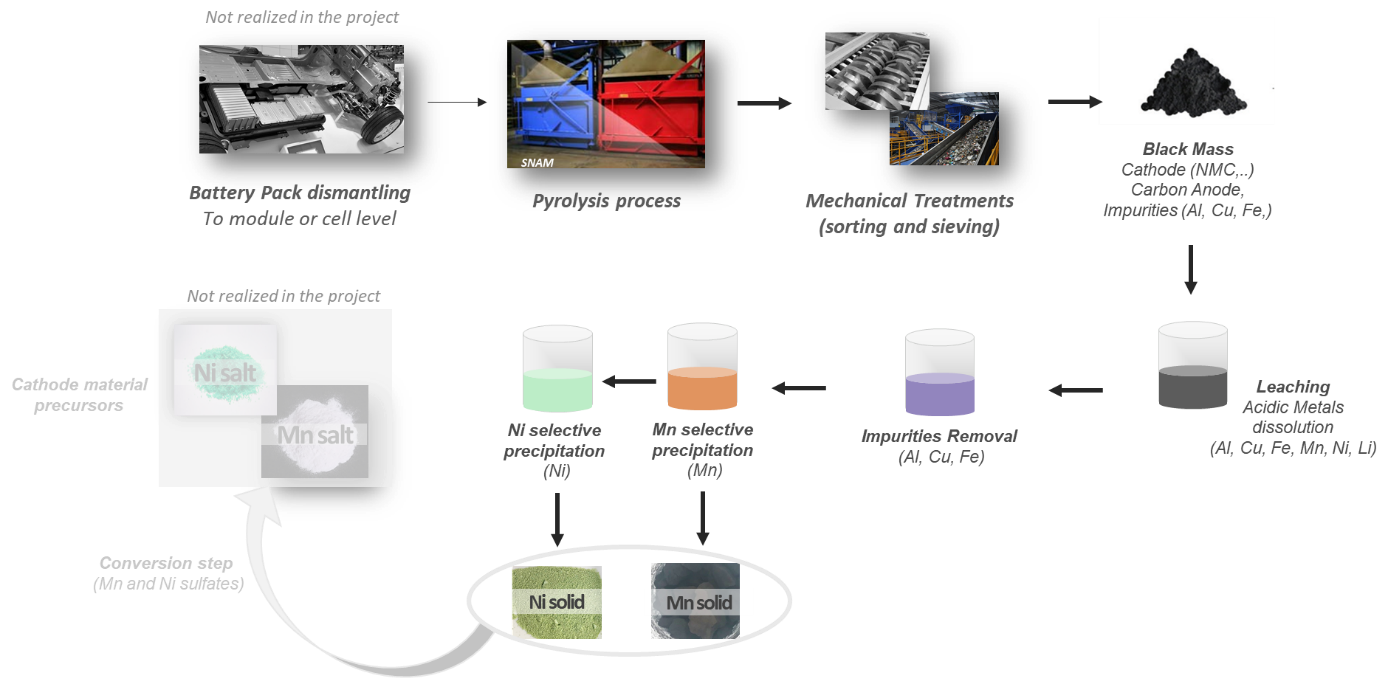
The main steps of the recycling process considered in the project are summarized in the figure above. First, the end-of-life battery packs are dismantled before applying thermolysis to the modules or cells to deactivate them and eliminate plastics and volatile compounds. Mechanical treatments like shredding and sieving are then performed to obtain a black mass composed of cathode and anode active materials (LiNi0.5Mn1.5O2, Graphite) and impurities (Al, Cu, Fe, etc.). The obtained black mass is leached in acidic conditions to dissolve the metals and retrieve an insoluble mix comprising mainly Graphite. The leachate is then purified by removing the impurities (mainly Al, Cu, Fe). Finally, separative chemistry is used to recover the most valuable metals precipitates one by one (Mn, Ni and Li). Compared to the more commonly recycled LiNixMnyCozO2 cathode material, LiNi0.5Mn1.5O2 cathode material does not contain Co, which simplifies the recovery steps of valuable metals, as Mn and Co are of close chemical behavior and thus are difficult to separate.
The first selective precipitation step used by CEA and SNAM is based on the Mn2+ oxidation to form a MnOOH precipitate. The selective precipitation conditions (pH, temperature, etc.) are optimized to maximize the Mn precipitation rate without generating Ni co-precipitation. After recovering the Mn, the Ni is separated from the leachate by basic precipitation. The project developments led to a recovery rate of 97 % for the Mn selective precipitation and 90 % for the Ni basic precipitation, with expected purities of more than 98 % and 99,9 % respectively, when considering only the valuable metals. These performances were demonstrated at lab scale at CEA and then confirmed at pilot scale at SNAM. The robustness of the 3beLieEVe recycling process could be tested even further by working with a large amount of material to recycle (at module or cell level), to refine the process conditions and make a complete assessment of the performance criteria.